


 and MM12928
and MM12928  .
.
Product name |
For dates of discharge |
Immunotherapy approach |
ICD-10 PCS code |
|---|---|---|---|
Obe-cel/Aucatzyl |
On and after November 8, 2024 |
Via peripheral vein |
XW0338A - Introduction of Obecabtagene autoleucel into peripheral vein, percutaneous approach, new technology group 10 |
Obe-cel/Aucatzyl |
On and after November 8, 2024 |
Through central vein |
XW0438A - Introduction of Obecabtagene autoleucel into central vein, percutaneous approach, new technology group 10 |
Carvykti |
On and after February 28, 2022 |
Via peripheral vein |
XW033A7 -- Carvykti: Introduction of ciltacabtagene autoleucel into peripheral vein, percutaneous approach, new technology group 7 |
Carvykti |
On and after February 28, 2022 |
Through central vein |
XW043A7 - Carvykti: Introduction of ciltacabtagene autoleucel into central vein, percutaneous approach, new technology group 7 |
Yescarta |
On and after October 1, 2021 |
Via peripheral vein |
XW033H7 -- Yescarta: Introduction of axicabtagene ciloleucel immunotherapy into peripheral vein, percutaneous approach, new technology group 7 |
Yescarta |
On and after October 1, 2021 |
Through central vein |
XW043H7 -- Yescarta: Introduction of axicabtagene ciloleucel immunotherapy into central vein, percutaneous approach, new technology group 7 |
Kymriah |
On and after October 1, 2021 |
Via peripheral vein |
XW033J7 -- Kymriah: Introduction of tisagenlecleucel immunotherapy into peripheral vein, percutaneous approach, new technology group 7 |
Kymriah |
On and after October 1, 2021 |
Through central vein |
XW043J7 -- Kymriah: Introduction of tisagenlecleucel immunotherapy into central vein, percutaneous approach, new technology group 7 |
ABECMA |
On and after October 1, 2021 |
Via peripheral vein |
XW033K7 -- ABECMA: Introduction of idecabtagene vicleucel immunotherapy into peripheral vein, percutaneous approach, new technology group 7 |
ABECMA |
On and after October 1, 2021 |
Through central vein |
XW043K7 -- ABECMA: Introduction of idecabtagene vicleucel immunotherapy into central vein, percutaneous approach, new technology group 7 |
Tecartus |
On and after October 1, 2021 |
Via peripheral vein |
XW033M7 -- Tecartus: Introduction of brexucabtagene autoleucel immunotherapy into peripheral vein, percutaneous approach, new technology group 7 |
Tecartus |
On and after October 1, 2021 |
Through central vein |
XW043M7 -- Tecartus: Introduction of brexucabtagene autoleucel immunotherapy into central vein, percutaneous approach, new technology group 7 |
Breyanzi |
On and after October 1, 2021 |
Via peripheral vein |
XW033N7 -- Breyanzi: Introduction of lisocabtagene maraleucel immunotherapy into peripheral vein, percutaneous approach, new technology group 7 |
Breyanzi |
On and after October 1, 2021 |
Through central vein |
XW043N7 -- Breyanzi: Introduction of lisocabtagene maraleucel immunotherapy into central vein, percutaneous approach, new technology group 7 |
FDA approved products awaiting their own PCS code and products used in qualifying clinical trials |
On and after October 1, 2021 |
Via peripheral vein |
XW033C7 -- FDA approved products awaiting their own PCS code: Introduction of autologous engineered chimeric antigen receptor t-cell immunotherapy into peripheral vein, percutaneous approach, new technology group 7 |
FDA approved products awaiting their own PCS code and products used in qualifying clinical trials |
On and after October 1, 2021 |
Through central vein |
XW043C7 -- FDA approved products awaiting their own PCS code: Introduction of autologous engineered chimeric antigen receptor t-cell immunotherapy into central vein, percutaneous approach, new technology group 7 |
 , MM13734
, MM13734  and change request (CR) 13926
and change request (CR) 13926  for details.
for details.
Procedure or drug product |
Applicable DOS |
CPT/ HCPCS |
Payable or Not payable |
Rationale |
Additional Notes |
|---|---|---|---|---|---|
The administration* |
Effective January 1, 2025-current |
38228* |
Payable in Part A and B outpatient. Not payable in ASC. |
CAR T-cell therapy is not allowed in an ASC. |
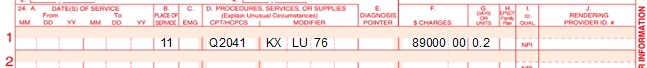
*Note: For Part B (outpatient claims), CPT code 38228 is only payable when the line item has a KX modifier appended. |
The administration* |
Effective August 7, 2019-December 31, 2024 |
0540T* |
Payable in Part A and B outpatient. Not payable in ASC. |
CAR T-cell therapy is not allowed in an ASC. |
*Note: For Part B (outpatient claims), CPT code 0540T is only payable when the line item has a KX modifier appended. |
Axicabtagene ciloleucel (Yescarta)* |
Effective August 7, 2019-current |
Q2041* |
Payable in Part A and B outpatient. Not payable in ASC. |
HCPCS code Q2041 has an ASC payment indicator "B5" (Alternative code may be available, no payment made). CAR T-cell therapy is not allowed in an ASC. |
*Note: For Part B (outpatient claims), HCPCS code Q2041 is only payable when the line item has a KX modifier appended. |
Tisagenlecleucel (Kymriah)* |
Effective August 7, 2019-current |
Q2042* |
Payable in Part A and B outpatient. Not payable in ASC. |
HCPCS code Q2042 has an ASC payment indicator "B5" (Alternative code may be available, no payment made). CAR T-cell therapy is not allowed in an ASC |
Note: For Part B (outpatient claims), HCPCS code Q2042 is only payable when the line item has a KX modifier appended. |
Brexucabtagene Autoleucel (Tecartus)* |
Effective April 1, 2021-current |
Q2053* |
Payable in Part A and B outpatient. Not payable in ASC. |
CAR T-cell therapy is not allowed in an ASC. HCPCS code Q2053 is invalid in the ASC setting. |
*Note: For Part B (outpatient claims), HCPCS code Q2053 is only payable when the line item has a KX modifier appended. |
Brexucabtagene Autoleucel (Tecartus)* |
Effective July 24, 2020-March 31, 2021 |
J3490, J3590, or J9999* |
Payable in Part B. Packaged in Part A outpatient. Code should not be billed by ASCs. |
Code is used by Part B providers (not ASC) to report this product. *Note: For Part B (outpatient claims), HCPCS codes J3490, J3590 and J9999 are only payable when the line item has a KX modifier appended. | |
Brexucabtagene Autoleucel (Tecartus) |
Effective January 1, 2021-March 31, 2021 |
C9073 |
Not payable in Part B. Payable in Part A outpatient. Not payable in ASC. |
HCPCS code C9073 has an ASC payment indicator "B5" (Alternative code may be available, no payment made). CAR T-cell therapy is not allowed in an ASC. |
HCPCS code is non-payable on Part B provider claims. Code is used by Part A outpatient and ASCs (not Part B providers) to report this product. |
Brexucabtagene Autoleucel (Tecartus) |
Effective July 24, 2020-December 31, 2020 |
C9399 |
Not payable in Part B. Payable in Part A outpatient. Not payable in ASC. |
CAR T-cell therapy is not allowed in an ASC. |
HCPCS code is non-payable on Part B provider claims. Code is used by Part A outpatient and ASCs (not Part B providers) to report this product. |
Lisocabtagene maraleucel (Breyanzi)* |
Effective October 1, 2021-current |
Q2054* |
Payable in Part A and B outpatient. Not payable in ASC. |
CAR T-cell therapy is not allowed in an ASC. HCPCS code Q2054 is invalid in the ASC setting. |
*Note: For Part B (outpatient claims), HCPCS code Q2054 is only payable when the line item has a KX modifier appended. |
Lisocabtagene maraleucel (Breyanzi)* |
Effective February 5, 2021-September 30, 2021 |
J3490, J3590, or J9999* |
Payable in Part B. Packaged in Part A outpatient. Code should not be billed by ASCs. |
Code is used by Part B providers (not ASC) to report this product. *Note: For Part B (outpatient claims), HCPCS codes J3490, J3590 and J9999 are only payable when the line item has a KX modifier appended. | |
Lisocabtagene maraleucel (Breyanzi) |
Effective July 1, 2021-September 30, 2021 |
C9076 |
Not payable in Part B. Payable in Part A outpatient. Not payable in ASC. |
CAR T-cell therapy is not allowed in an ASC. HCPCS code C9076 is invalid in the ASC setting. |
HCPCS code is non-payable on Part B provider claims. Code is used by Part A outpatient and ASCs (not Part B providers) to report this product. |
Lisocabtagene maraleucel (Breyanzi) |
Effective February 5, 2021-June 30, 2021 |
C9399 |
Not payable in Part B. Payable in Part A outpatient. Not payable in ASC. |
CAR T-cell therapy is not allowed in an ASC. |
HCPCS code is non-payable on Part B provider claims. Code is used by Part A outpatient and ASCs (not Part B providers) to report this product. |
Idecabtagene vicleucel (Abecma)* |
Effective January 1, 2022-current |
Q2055* |
Payable in Part A and B outpatient. Not payable in ASC. |
CAR T-cell therapy is not allowed in an ASC. HCPCS code Q2055 is invalid in the ASC setting. |
*Note: For Part B (outpatient claims), HCPCS code Q2055 is only payable when the line item has a KX modifier appended. |
Idecabtagene vicleucel (Abecma)* |
Effective March 26, 2021-December 31, 2021 |
J3490, J3590, or J9999* |
Payable in Part B. Packaged in Part A outpatient. Code should not be billed by ASCs. |
Code is used by Part B providers (not ASC) to report this product. *Note: For Part B (outpatient claims), HCPCS codes J3490, J3590 and J9999 are only payable when the line item has a KX modifier appended. | |
Idecabtagene vicleucel (Abecma) |
Effective October 1, 2021-December 31, 2021 |
C9081 |
Not payable in Part B. Payable in Part A outpatient. Not payable in ASC. |
HCPCS code C9081 has an ASC payment indicator "B5" (Alternative code may be available, no payment made) CAR T-cell therapy is not allowed in an ASC. |
HCPCS code is non-payable on Part B provider claims. Code is used by Part A outpatient and ASCs (not Part B providers) to report this product. |
Idecabtagene vicleucel (Abecma) |
Effective March 21, 2021-September 30, 2021 |
C9399 |
Not payable in Part B. Payable in Part A outpatient. Not payable in ASC. |
CAR T-cell therapy is not allowed in an ASC. |
HCPCS code is non-payable on Part B provider claims. Code is used by Part A outpatient and ASCs (not Part B providers) to report this product. |
Ciltacabtagene autoleucel (Carvykti)* |
Effective October 1, 2022-current |
Q2056* |
Payable in Part A and B outpatient. Not payable in ASC. |
CAR T-cell therapy is not allowed in an ASC. HCPCS code Q2056 is invalid in the ASC setting. |
*Note: For Part B (outpatient claims), HCPCS code Q2056 is only payable when the line item has a KX modifier appended. |
Ciltacabtagene autoleucel (Carvykti) |
Effective July 1, 2022-September 30, 2022 |
C9098 |
Not payable in Part B. Payable in Part A outpatient. Not payable in ASC. |
CAR T-cell therapy is not allowed in an ASC. HCPCS code C9098 is invalid in the ASC setting. |
HCPCS code is non-payable on Part B provider claims. Code is used by Part A outpatient and ASCs (not Part B providers) to report this product. |
Ciltacabtagene autoleucel (Carvykti) |
Effective February 28, 2022- June 30, 2022 |
C9399 |
Not payable in Part B. Payable in Part A outpatient. Not payable in ASC. |
CAR T-cell therapy is not allowed in an ASC. |
HCPCS code is non-payable on Part B provider claims. Code is used by Part A outpatient and ASCs (not Part B providers) to report this product. |
Ciltacabtagene autoleucel (Carvykti)* |
Effective February 28, 2022- September 30, 2022 |
J3490, J3590, or J9999* |
Payable in Part B. Packaged in Part A outpatient. Code should not be billed by ASCs. |
Code is used by Part B providers (not ASC) to report this product. *Note: For Part B (outpatient claims), HCPCS codes J3490, J3590 and J9999 are only payable when the line item has a KX modifier appended. | |
Obe-cel/ AUCATZYL« (Obecabtagene Autoleucel) |
Effective July 1, 2025 - current |
Q2058* |
Payable in Part A and B outpatient. Not payable in ASC. |
CAR T-cell therapy is not allowed in an ASC. HCPCS code Q2058 is invalid in the ASC setting. |
*Note: For Part B (outpatient claims), HCPCS code Q2058 is only payable when the line item has a KX modifier appended. |
Obe-cel/ AUCATZYL« (Obecabtagene Autoleucel) |
Effective April 1, 2025 - June 30, 2025 |
C9301 |
Not payable in Part B. Payable in Part A outpatient. Not payable in ASC |
CAR T-cell therapy is not allowed in an ASC. |
HCPCS code is non-payable on Part B provider claims. Code is used by Part A outpatient and ASCs (not Part B providers) to report this product. |
Obe-cel/ AUCATZYL« (Obecabtagene Autoleucel) |
Effective November 8, 2024 – March 31, 2025 |
C9399 |
Not payable in Part B. Payable in Part A outpatient. Not payable in ASC |
CAR T-cell therapy is not allowed in an ASC. |
HCPCS code is non-payable on Part B provider claims. Code is used by Part A outpatient and ASCs (not Part B providers) to report this product. |
Obe-cel/ AUCATZYL« (Obecabtagene Autoleucel) |
Effective November 8, 2024 – June 30, 2025 |
J3490, J3590, J9999* |
Payable in Part B. Packaged in Part A outpatient. Code should not be billed by ASCs. |
Code is used by Part B providers (not ASC) to report this product. *Note: For Part B (outpatient claims), HCPCS codes J3490, J3590, J9999 are only payable when the line item has a KX modifier appended. | |
Collection/Handling** |
Effective January 1, 2025-current |
38225** |
Not payable |
**Tracking codes only. These steps are not paid separately. |
**CPT code represents steps required to collect and prepare the genetically modified T-cells. |
Collection/Handling** |
Effective August 7, 2019-December 31, 2024 |
0537T** |
Not payable |
**Tracking codes only. These steps are not paid separately. |
**CPT code represents steps required to collect and prepare the genetically modified T-cells. |
Preparation for transport** |
Effective January 1, 2025-current |
38226** |
Not payable |
**Tracking codes only. These steps are not paid separately. |
**CPT code represents steps required to collect and prepare the genetically modified T-cells. |
Preparation for transport** |
Effective August 7, 2019-December 31, 2024 |
0538T** |
Not payable |
**Tracking codes only. These steps are not paid separately. |
**CPT code represents steps required to collect and prepare the genetically modified T-cells. |
Receipt and preparation** |
Effective January 1, 2025-current |
38227** |
Not payable |
**Tracking codes only. These steps are not paid separately. |
**CPT code represents steps required to collect and prepare the genetically modified T-cells. |
Receipt and preparation** |
Effective August 7, 2019-December 31, 2024 |
0539T** |
Not payable |
**Tracking codes only. These steps are not paid separately. |
**CPT code represents steps required to collect and prepare the genetically modified T-cells. |

 . Make sure your billing staff are aware of these changes if you bill for these services.
. Make sure your billing staff are aware of these changes if you bill for these services.  ;
;  ; CR13926 Fiscal Intermediary Shared System (FISS) Changes to Automate the Application of Condition Code ZC for Chimeric Antigen Receptor (CAR) T-Cell and Other Immunotherapy Cases Involving a Clinical
; CR13926 Fiscal Intermediary Shared System (FISS) Changes to Automate the Application of Condition Code ZC for Chimeric Antigen Receptor (CAR) T-Cell and Other Immunotherapy Cases Involving a Clinical ; CMS IOM Pub. 100-04, Chapter 32, Section 400
; CMS IOM Pub. 100-04, Chapter 32, Section 400  ; CMS NCD 110.24 CAR
; CMS NCD 110.24 CAR ; MM12177 NCD 110.24: Chimeric Antigen Receptor (CAR) T-cell Therapy - This change request (CR) Rescinds and Fully Replaces CR 11783
; MM12177 NCD 110.24: Chimeric Antigen Receptor (CAR) T-cell Therapy - This change request (CR) Rescinds and Fully Replaces CR 11783  ; CR12245 October 2021 HCPCS Quarterly Update Reminder
; CR12245 October 2021 HCPCS Quarterly Update Reminder  ;
; ;
; ; MM12480 ICD-10 and Other Coding Revisions to NCDs -- April Added to sources: 2022 (CR 1 of 2)
; MM12480 ICD-10 and Other Coding Revisions to NCDs -- April Added to sources: 2022 (CR 1 of 2)  ;
; ; CMS IOM Pub. 100-04 Medicare Claims Processing Manual, Chapter 32, section 68-68.4
; CMS IOM Pub. 100-04 Medicare Claims Processing Manual, Chapter 32, section 68-68.4 