Last Modified: 4/20/2024
Location: FL, PR, USVI
Business: Part B
The reporting of the national drug code (NDC) number is not required on Medicare claims unless the beneficiary is dually eligible for Medicare and Medicaid. The Deficit Reduction Act of 2005 required State Medicaid agencies to provide for the collection of NDCs on all claims for certain physician-administered drugs for the purpose of billing manufacturers for Medicaid drug rebates.
To capture the information needed to fulfill the rebate requirements, Medicare providers billing for dual eligible patients will be required to submit the NDCs for physician-administered drugs.
Ensure dosage administered is accurately reported in terms of units specified in HCPCS code descriptor:
• Report unit(s) in multiples based on HCPCS code descriptor.
• If the units listed in the "J" code descriptor can be multiplied to reflect administered dosage.
• Bill on one line and use “J” code, with the appropriate number of units which reflect dosage given.
• Example 1:
• J2357 HCPCS code description is Injection, omalizumab, 5 mg.
• One unit of code J2357 represents 5 mg ordered or administered per patient.
• Code J2357 is billed based on units, not the total number of milligrams.
• When the quantity of omalizumab administered is 30 mg with the code description of the drug code J2357 specifying one unit is 5 mg, the units billed should be six, not the total number of milligrams administered.
• It is not appropriate to use "J" code with multiplier in the unit field, when there is another "J" code that more closely describes amount given.
• Example 2:
• J1020 HCPCS code description is injection, methylprednisolone, 20 mg.
• J1030 HCPCS code description is injection, methylprednisolone, 40 mg.
• The provider administered 40 mg of methylprednisolone. Report HCPCS code J1030 with a unit of one.
When required to submit NDCs, drug and quantity information for Medicaid rebates:
• Submit the NDC code in the red shaded portion of the detail line (Item 24) in position 01 through position 13 or the electronic equivalent.
• The NDC is to be preceded with the qualifier N4 and followed immediately by the 11-digit NDC code.
• There are six characters available for quantity. Report the NDC quantity in positions 17 through 24 of the same, red-shaded section.
The quantity is to be preceded by the appropriate qualifier:
• UN (units)
• F2 (international units)
• GR (gram)
• ME (milligram)
• ML (milliliter)
If the quantity is less than six characters, left justify and space-fill the remaining positions (e.g., UN2 or F2999999).
• Example 3:
Patient received 30 mg of J2357 (injection, omalizumab, 5 mg) report the following:
• J2357 HCPCS code description injection, omalizumab, 5 mg.
• One unit represents 5 mg ordered or administered per patient.
• Code J2357 is billed based on units, not the total number of milligrams.
• Calculate:
• 30 mg administered ÷ 5 mg per code description = 6 units.
• Use the NDC that closely matches the units administered.
• The following NDC's represent code J2357:
• NDC 50242021501 = 30
• NDC 50242021401 = 15
Paper claims:
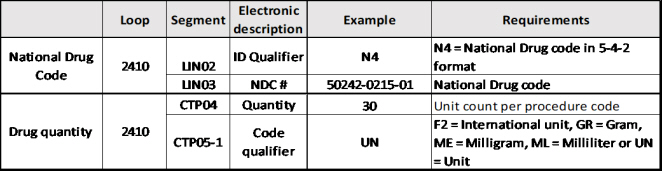
Electronic claims:
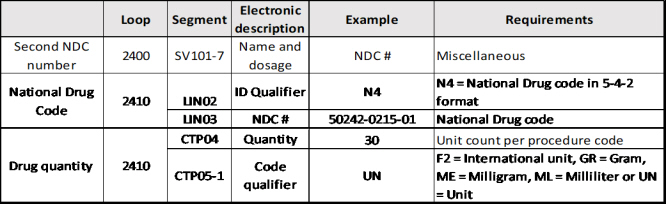
For each line level reporting of a Part B physician-administered drug, continue to report the associated HCPCS code (e.g., J3140) in 2400 SV102-2, with SV102-1 HC:
• For each Part B drug HCPCS code reported in 2400 SV102-2, complete the required associated 2410 LIN and CPT04 segments as follows:
• Include the NDC in 2410 LIN03, with LIN02=N4;
• Include the quantity/unit count in 2410 CTP04; and
• Input the needed information in 2410 CTP05 and CTP05-1.
When providers need to report two NDC numbers for one drug, the additional NDC should be reported in item 19 of the CMS-1500 claim form or the electronic equivalent. Do not report multiple lines of the same drug. It will result in duplicate denials and subsequent appeals.
• Example 4:
Patient received 45 mg of J2357 (injection, omalizumab, 5 mg) report the following:
• J2357 HCPCS description is injection, omalizumab, 5 mg.
• One unit represents 5 mg ordered/administered per patient.
• Code J2357 is billed based on units, not the total number of milligrams.
• Calculate:
• 45 mg administered ÷ 5 mg per description = 9 units.
• Use the NDC that closely matches the units administered.
• The following NDC's represent J2357:
• NDC 50242021501 = 30
• NDC 50242021401 = 15
Paper claims:
Electronic claims:
First Coast Service Options (First Coast) strives to ensure that the information available on our provider website is accurate, detailed, and current. Therefore, this is a dynamic site and its content changes daily. It is best to access the site to ensure you have the most current information rather than printing articles or forms that may become obsolete without notice.