Home ►
Billing news ►
Kisunla for monoclonal antibodies directed against amyloid for the treatment of Alzheimer's disease
Last Modified: 7/15/2024
Location: FL, PR, USVI
Business: Part A, Part B
Effective for dates of service on and after July 2, Medicare will pay for Kisunla for monoclonal antibodies directed against amyloid for the treatment of Alzheimer's disease.
Medicare covers the drugs with traditional FDA approval in this class when a prescribing clinician or their staff decides the Medicare coverage criteria is met. The clinician or staff also submits information to help answer treatment questions in a qualifying study. You can participate in the CMS
National Patient Registry 
(or another CMS-approved study) to get Medicare payment for treating your patients with Kisunla.
Institutional claims:
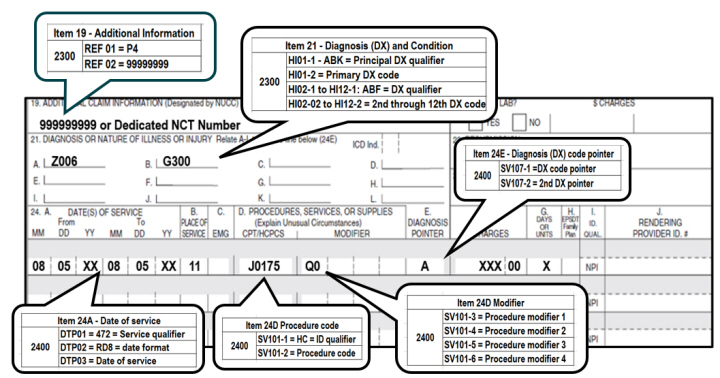
• For dates or dates of service on or after July 2, 2024, use HCPCS code J0175, Kisunla, (Injection, donanemab-azbt, 2 mg)
• Type of Bill: 12X, 13X, or 85X
• Revenue code: 0636
• Condition code: 30
• Value code: D4 with the National Clinical Trail (NCT) Number "99999999" or the dedicated NCT Number.
• Report one the following modifiers:
• Q0 (Investigational clinical service provided in a clinical research study that is in an approved clinical research study), or
• Q1 (Routine clinical service provided in a clinical research study that is in an approved clinical research study)
• Diagnosis codes:
• Z00.6 (noting a registry) AND one of the following dx codes:
• G30.0 Alzheimer's disease w/early onset
• G30.1 Alzheimer's disease w/late onset
• G30.8 Other Alzheimer's disease
• G30.9 Alzheimer's disease, unspecified
• G31.84 mild cognitive impairment
• For dates of service on or after July 2, 2024, use HCPCS code J0175, Kisunla, (Injection, donanemab-azbt, 2 mg)
• Report the NCT (8-digit number) "99999999" or the dedicated NCT Number in the narrative description field (Item 19) or the electronic claim in Loop 2300 REF02 (REF01=P4).
• To ensure claims are submitted and processed correctly, please review the reporting information with your software vendor.
• Click here to view the CMS-1500 (02/12) data element requirements and electronic claim loop mapping.
• Report one of the following modifiers:
• Q0 (Investigational clinical service provided in a clinical research study that is in an approved clinical research study), or
• Q1 (Routine clinical service provided in a clinical research study that is in an approved clinical research study)
• Diagnosis codes:
• Z00.6 (noting a registry) AND one of the following dx codes:
• G30.0 Alzheimer's disease w/early onset
• G30.1 Alzheimer's disease w/late onset
• G30.8 Other Alzheimer's disease
• G30.9 Alzheimer's disease, unspecified
• G31.84 mild cognitive impairment
• The diagnosis code pointer should be used to indicate the primary diagnosis on the claim form.
First Coast Service Options (First Coast) strives to ensure that the information available on our provider website is accurate, detailed, and current. Therefore, this is a dynamic site and its content changes daily. It is best to access the site to ensure you have the most current information rather than printing articles or forms that may become obsolete without notice.



 .
. (or another CMS-approved study) to get Medicare payment for treating your patients with Kisunla.
(or another CMS-approved study) to get Medicare payment for treating your patients with Kisunla.