page.
page.
Code |
Description |
Labeler name |
Vaccine/procedure name*** |
Effective date |
|---|---|---|---|---|
91300* |
SARSCOV2 VAC 30MCG/0.3ML IM |
Pfizer |
Pfizer-BioNTech COVID-19 Vaccine (Aged 12 years and older) (Purple Cap) |
12/11/2020-04/18/2023 |
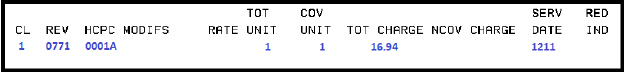
0001A |
ADM SARSCOV2 30MCG/0.3ML 1ST |
Pfizer |
Pfizer-BioNTech COVID-19 Vaccine (Purple Cap) Administration – First Dose |
12/11/2020-04/18/2023 |
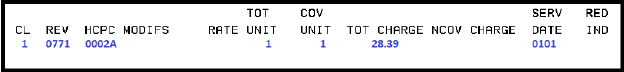
0002A |
ADM SARSCOV2 30MCG/0.3ML 2ND |
Pfizer |
Pfizer-BioNTech COVID-19 Vaccine (Purple Cap) Administration – Second Dose |
12/11/2020-04/18/2023 |
0003A |
ADM SARSCOV2 30MCG/0.3ML 3RD |
Pfizer |
Pfizer-BioNTech COVID-19 Vaccine (Purple Cap) Administration – Third Dose |
08/12/2021-04/18/2023 |
0004A |
ADM SARSCOV2 30MCG/0.3ML BST |
Pfizer |
Pfizer-BioNTech COVID-19 Vaccine (Purple Cap) Administration – Booster |
09/22/2021-04/18/2023 |
91301* |
SARSCOV2 VAC 100MCG/0.5ML IM |
Moderna |
Moderna COVID-19 Vaccine (Aged 12 years and older) (Red Cap) |
12/18/2020-04/18/2023 |
91318 |
SARSCOV2 VAC 3MCG TRS-SUC IM |
Pfizer |
Pfizer-BioNTech COVID-19 Vaccine 2023-2024 Formula (Yellow Cap) |
09/11/2023 |
91319 |
SARSCV2 VAC 10MCG TRS-SUC IM |
Pfizer |
Pfizer-BioNTech COVID-19 Vaccine 2023-2024 Formula (Blue Cap) |
09/11/2023 |
91320 |
SARSCV2 VAC 30MCG TRS-SUC IM |
Pfizer |
COMIRNATY (COVID-19 Vaccine, mRNA) 2023-2024 Formula |
09/11/2023 |
91321 |
SARSCOV2 VAC 25 MCG/.25ML IM |
Moderna |
Moderna COVID-19 Vaccine 2023-2024 Formula |
09/11/2023 |
91322 |
SARSCOV2 VAC 50 MCG/0.5ML IM |
Moderna |
SPIKEVAX 2023-2024 Formula |
09/11/2023 |
0011A |
ADM SARSCOV2 100MCG/0.5ML1ST |
Moderna |
Moderna COVID-19 Vaccine (Red Cap) Administration – First Dose |
12/18/2020-04/18/2023 |
0012A |
ADM SARSCOV2 100MCG/0.5ML2ND |
Moderna |
Moderna COVID-19 Vaccine (Red Cap) Administration – Second Dose |
12/18/2020-04/18/2023 |
0013A |
ADM SARSCOV2 100MCG/0.5ML3RD |
Moderna |
Moderna COVID-19 Vaccine (Red Cap) Administration – Third Dose |
08/12/2021-04/18/2023 |
91303* |
SARSCOV2 VAC AD26 .5ML IM |
Janssen |
Janssen COVID-19 Vaccine (Aged 18 years and older) |
02/27/2021-06/01/2023 |
0031A |
ADM SARSCOV2 VAC AD26 .5ML |
Janssen |
Janssen COVID-19 Vaccine Administration – First Dose |
02/27/2021-06/01/2023 |
0034A |
ADM SARSCOV2 VAC AD26 .5ML B |
Janssen |
Janssen COVID-19 Vaccine Administration - Booster |
10/20/2021-06/01/2023 |
0044A |
ADM SARSCOV2 5MCG/0.5ML BST |
Novavax |
Novavax COVID-19 Vaccine Administration - Booster |
10/19/2022-10/03/2023 |
91304* |
SARSCOV2 VAC 5MCG/0.5ML IM |
Novavax |
Novavax COVID-19 Vaccine, Adjuvanted (Aged 12 years and older) |
07/13/2022 |
0041A |
ADM SARSCOV2 5MCG/0.5ML 1ST |
Novavax |
Novavax COVID-19 Vaccine, Adjuvanted Administration – First Dose |
07/13/2022-10/03/2023 |
0042A |
ADM SARSCOV2 5MCG/0.5ML 2ND |
Novavax |
Novavax COVID-19 Vaccine, Adjuvanted Administration – Second Dose |
07/13/2022-10/03/2023 |
91305* |
SARSCOV2 VAC 30 MCG TRS-SUCR |
Pfizer |
Pfizer-BioNTech Covid-19 Vaccine Pre-Diluted (Aged 12 years and older) (Gray Cap) |
01/03/2022-04/18/2023 |
0051A |
ADM SARSCV2 30MCG TRS-SUCR 1 |
Pfizer |
Pfizer-BioNTech COVID-19 Vaccine Pre-Diluted (Gray Cap) Administration - First dose |
01/03/2022-04/18/2023 |
0052A |
ADM SARSCV2 30MCG TRS-SUCR 2 |
Pfizer |
Pfizer-BioNTech COVID-19 Vaccine Pre-Diluted (Gray Cap) Administration - Second dose |
01/03/2022-04/18/2023 |
0053A |
ADM SARSCV2 30MCG TRS-SUCR 3 |
Pfizer |
Pfizer-BioNTech COVID-19 Vaccine Pre-Diluted (Gray Cap) Administration - Third dose |
01/03/2022-04/18/2023 |
0054A |
ADM SARSCV2 30MCG TRS-SUCR B |
Pfizer |
Pfizer-BioNTech COVID-19 Vaccine Pre-Diluted (Gray Cap) Administration - Booster |
01/03/2022-04/18/2023 |
91306* |
SARSCOV2 VAC 50MCG/0.25ML IM |
Moderna |
Moderna COVID-19 Vaccine (Aged 18 years and older) (Red Cap) (Low Dose) |
10/20/2021-04/18/2023 |
0064A |
ADM SARSCOV2 50MCG/0.25MLBST |
Moderna |
Moderna COVID-19 Vaccine (Red Cap) (Low Dose) Administration - Booster |
10/20/2021-04/18/2023 |
91307* |
SARSCOV2 VAC 10 MCG TRS-SUCR |
Pfizer |
Pfizer-BioNTech COVID-19 Pediatric Vaccine (Aged 5 years through 11 years) (Orange Cap) |
10/29/2021-04/18/2023 |
0071A |
ADM SARSCV2 10MCG TRS-SUCR 1 |
Pfizer |
Pfizer-BioNTech COVID-19 Pediatric Vaccine (Orange Cap) - Administration - First dose |
10/29/2021-04/18/2023 |
0072A |
ADM SARSCV2 10MCG TRS-SUCR 2 |
Pfizer |
Pfizer-BioNTech Covid-19 Pediatric Vaccine (Orange Cap) - Administration - Second dose |
10/29/2021-04/18/2023 |
0073A |
ADM SARSCV2 10MCG TRS-SUCR 3 |
Pfizer |
Pfizer-BioNTech COVID-19 Pediatric Vaccine (Orange Cap) - Administration - Third dose |
01/03/2022-04/18/2023 |
0074A |
ADM SARSCV2 10MCG TRS-SUCR B |
Pfizer |
Pfizer-BioNTech COVID-19 Pediatric Vaccine (Orange Cap) - Administration - Booster |
05/17/2022-04/18/2023 |
91308* |
SARSCOV2 VAC 3MCG TRS-SUCR |
Pfizer |
Pfizer-BioNTech COVID-19 Pediatric Vaccine (Aged 6 months through 4 years) (Maroon Cap) |
06/17/2022-04/18/2023 |
0081A |
ADM SARSCOV2 3MCG TRS-SUCR 1 |
Pfizer |
Pfizer-BioNTech COVID-19 Pediatric Vaccine (Aged 6 months through 4 years) (Maroon Cap) - Administration - First dose |
06/17/2022-04/18/2023 |
0082A |
ADM SARSCOV2 3MCG TRS-SUCR 2 |
Pfizer |
Pfizer-BioNTech COVID-19 Pediatric Vaccine (Aged 6 months through 4 years) (Maroon Cap) - Administration - Second dose |
06/17/2022-04/18/2023 |
0083A |
ADM SARSCOV2 3MCG TRS-SUCR 3 |
Pfizer |
Pfizer-BioNTech COVID-19 Pediatric Vaccine (Aged 6 months through 4 years) (Maroon Cap) - Administration - Third dose |
06/17/2022-04/18/2023 |
91309* |
SARSCOV2 VAC 50MCG/0.5ML IM |
Moderna |
Moderna COVID-19 Vaccine (Aged 6 years through 11 years or aged 18 years and older) (Blue Cap with purple border) 50MCG/0.5ML |
03/29/2022-04/18/2023 |
0091A |
ADM SARSCOV2 50 MCG/.5 ML1ST |
Moderna |
Moderna COVID-19 Pediatric Vaccine (Aged 6 years through 11 years) (Blue Cap with purple border) - Administration - First dose |
06/17/2022-04/18/2023 |
0092A |
ADM SARSCOV2 50 MCG/.5 ML2ND |
Moderna |
Moderna COVID-19 Pediatric Vaccine (Aged 6 years through 11 years) (Blue Cap with purple border) - Administration - Second dose |
06/17/2022-04/18/2023 |
0093A |
ADM SARSCOV2 50 MCG/.5 ML3RD |
Moderna |
Moderna COVID-19 Pediatric Vaccine (Aged 6 years through 11 years) (Blue Cap with purple border) - Administration - Third dose |
06/17/2022-04/18/2023 |
0094A |
ADM SARSCOV2 50MCG/0.5 MLBST |
Moderna |
Moderna COVID-19 Vaccine (Aged 18 years and older) (Blue Cap with purple border) 50MCG/0.5ML Administration - Booster |
03/29/2022-04/18/2023 |
91311* |
SARSCOV2 VAC 25MCG/0.25ML IM |
Moderna |
Moderna COVID-19 Pediatric Vaccine (Aged 6 months through 5 years) (Blue Cap with magenta border) 250MCG/0.25ML |
06/17/2022-04/18/2023 |
0111A |
ADM SARSCOV2 25MCG/0.25ML1ST |
Moderna |
Moderna COVID-19 Pediatric Vaccine (Aged 6 months through 5 years) (Blue Cap with magenta border) - Administration - First dose |
06/17/2022-04/18/2023 |
0112A |
ADM SARSCOV2 25MCG/0.25ML2ND |
Moderna |
Moderna COVID-19 Pediatric Vaccine (Aged 6 months through 5 years) (Blue Cap with magenta border) - Administration - Second dose |
06/17/2022-04/18/2023 |
0121A |
ADM SARSCV2 BVL 30MCG/.3ML 1 |
Pfizer |
1st dose Pfizer Pfizer-BioNTech COVID-19 Vaccine, Bivalent (Age 12 years and older). Single dose. |
04/18/2023-09/12/2023 |
0141A |
ADM SRSCV2 BVL 25MCG/.25ML 1 |
Moderna |
1st dose Moderna COVID-19 Vaccine, Bivalent (Age 6 months to 11 years old). First dose. |
04/18/2023-09/12/2023 |
0142A |
ADM SRSCV2 BVL 25MCG/.25ML 2 |
Moderna |
2nd dose Moderna COVID-19 Vaccine, Bivalent (Age 6 months to 11 years old). Single dose. |
04/18/2023-09/12/2023 |
0151A |
ADM SARSCV2 BVL 10MCG/.2ML 1 |
Pfizer |
1st dose Pfizer Pfizer-BioNTech COVID-19 Vaccine, Bivalent (Age 5 years through 11 years old). Single dose. |
04/18/2023-09/12/2023 |
0171A |
ADM SARSCV2 BVL 3MCG/0.2ML 1 |
Pfizer |
1st dose Pfizer Pfizer-BioNTech COVID-19 Vaccine, Bivalent (Age 6 months through 4 years old). First dose. |
04/18/2023-09/12/2023 |
0172A |
ADM SARSCV2 BVL 3MCG/0.2ML 2 |
Pfizer |
2nd dose Pfizer Pfizer-BioNTech COVID-19 Vaccine, Bivalent (Age 6 months through 4 years old) |
04/18/2023-09/12/2023 |
91312 |
SARSCOV2 VAC BVL 30MCG/0.3M |
Pfizer |
Pfizer-BioNTech COVID-19 Vaccine, Bivalent Product (Aged 12 years and older) (Gray Cap) |
08/31/2022-09/12/2023 |
0124A |
ADM SARSCV2 BVL 30MCG/.3ML B |
Pfizer |
Pfizer-BioNTech COVID-19 Vaccine, Bivalent (Gray Cap) Administration – Additional dose |
08/31/2022-09/12/2023 |
91313 |
SARSCOV2 VAC BVL 50MCG/0.5ML |
Moderna |
Moderna COVID-19 Vaccine, Bivalent Product (Aged 18 years and older) (Dark Blue Cap with gray border). Additional dose. |
08/31/2022-09/12/2023 |
0134A |
ADM SARSCV2 BVL 50MCG/.5ML B |
Moderna |
Moderna COVID-19 Vaccine, Bivalent (Aged 18 years and older) (Dark Blue Cap with gray border) Administration – Additional Dose |
08/31/2022-09/12/2023 |
0113A |
ADM SARSCOV2 25MCG/0.25ML3RD |
Moderna |
Moderna COVID-19 Pediatric Vaccine (Aged 6 months through 5 years) (Blue Cap with magenta border) - Administration - Third dose |
06/17/2022 |
91314 |
SARSCOV2 VAC BVL 25MCG/.25ML |
Moderna |
Moderna COVID-19 Vaccine, Bivalent product (Age 6 months through 11 years). Additional dose. |
10/12/2022-09/12/2023 |
0144A |
COVID-19 Vaccine, Bivalent - Administration |
Moderna |
Moderna COVID-19 Vaccine, Bivalent - Administration – Additional Dose |
10/12/2022-09/12/2023 |
91315 |
SARSCOV2 VAC BVL 10MCG/0.2ML |
Pfizer |
Pfizer-BioNTech COVID-19 Vaccine, Bivalent product |
10/12/2022-09/12/2023 |
0154A |
COVID-19 Vaccine, Bivalent - Administration |
Pfizer |
Pfizer COVID-19 Vaccine, Bivalent - Administration – Additional dose |
10/12/2022-09/12/2023 |
91316 |
SARSCOV2 VAC BVL 10MCG/0.2ML |
Moderna |
Moderna COVID-19 Vaccine, Bivalent for use as a booster for ages 6 months through 5 years. Additional dose. |
12/08/2022-09/12/2023 |
0164A |
COVID-19 Vaccine, Bivalent Administration |
Moderna |
Moderna COVID-19 Vaccine, Bivalent Administration for use as a booster for ages 6 months through 5 years. Additional dose. |
12/08/2022-09/12/2023 |
91317 |
SARSCOV2 VAC BVL 3MCG/0.2ML |
Pfizer |
Pfizer-BioNTech COVID-19 Vaccine, Bivalent for use as a third primary series dose for ages 6 months through 4 years |
12/08/2022-09/12/2023 |
0172A |
ADM SARSCV2 BVL 3MCG/0.2ML 2 |
Pfizer |
Pfizer-BioNTech COVID-19 Vaccine, Bivalent for individuals 6 months through 4 years old. Second dose. |
04/18/2023-09/12/2023 |
0173A |
COVID-19 Vaccine, Bivalent Administration |
Pfizer |
Pfizer-BioNTech COVID-19 Vaccine, Bivalent |
12/08/2022-09/12/2023 |
0174A |
Pfizer-BioNtech COVID-19 Vaccine, Bivalent – Booster Dose |
Pfizer |
Pfizer-BioNTech COVID-19 Vaccine, Bivalent administration for use as a third primary series dose for ages 6 months through 4 years. Additional dose. |
03/14/2023-09/12/2023 |
90480 |
ADMN SARSCOV2 VACC 1 DOSE |
N/A |
Immunization administration by intramuscular injection of severe acute respiratory syndrome coronavirus 2 (SARSCoV-2) (coronavirus disease [COVID-19]) vaccine, single dose |
09/11/2023 |
M0201** |
COVID-19 vaccine home admin |
N/A |
COVID-19 vaccine administration inside a patient’s home; reported only once per individual home per date of service when only COVID-19 vaccine administration is performed at the patient’s home |
06/08/2021 |
Q0224 |
Inj, pemivibart, 4500 mg |
N/A |
Injection, pemivibart, for the pre-exposure prophylaxis only, for certain adults and adolescents (12 years of age and older weighing at least 40 kg) with no known SARS-CoV-2 exposure, and who either have moderate-to-severe immune compromise due to a medical condition or receipt of immunosuppressive medications or treatments, and are unlikely to mount an adequate immune response to COVID-19 vaccination, 4500 mg |
03/22/2024 |
M0224 |
Pemivibart infusion |
N/A |
Intravenous infusion, pemivibart, for the pre-exposure prophylaxis only, for certain adults and adolescents (12 years of age and older weighing at least 40 kg) with no known SARS-CoV-2 exposure, who either have moderate-to-severe immune compromise due to a medical condition or receipt of immunosuppressive medications or treatments, includes infusion and post administration monitoring |
03/22/2024 |
 .
. .
.
Code |
Description |
Labeler name |
Vaccine/procedure name |
Effective date |
|---|---|---|---|---|
Q0220* |
Tixagev and cilgav, 300mg |
AstraZeneca |
Injection, tixagevimab and cilgavimab, for the pre-exposure prophylaxis only, for certain adults and pediatric individuals (12 years of age and older weighing at least 40kg) with no known sars-cov-2 exposure, who either have moderate to severely compromised immune systems or for whom vaccination with any available COVID-19 vaccine is not recommended due to a history of severe adverse reaction to a COVID-19 vaccine(s) and/or COVID-19 vaccine component(s), 300 mg |
12/08/2021 – 01/26/2023 |
Q0221* |
Tixagev and cilgav, 600mg |
AstraZeneca |
Injection, tixagevimab and cilgavimab, for the pre-exposure prophylaxis only, for certain adults and pediatric individuals (12 years of age and older weighing at least 40kg) with no known sars-cov-2 exposure, who either have moderate to severely compromised immune systems or for whom vaccination with any available COVID-19 vaccine is not recommended due to a history of severe adverse reaction to a COVID-19 vaccine(s) and/or COVID-19 vaccine component(s), 600 mg |
02/24/2022 – 01/26/2023 |
M0220 |
Tixagev and cilgav inj |
AstraZeneca |
Injection, tixagevimab and cilgavimab, for the pre-exposure prophylaxis only, for certain adults and pediatric individuals (12 years of age and older weighing at least 40kg) with no known sars-cov-2 exposure, who either have moderate to severely compromised immune systems or for whom vaccination with any available COVID-19 vaccine is not recommended due to a history of severe adverse reaction to a COVID-19 vaccine(s) and/or COVID-19 vaccine component(s), includes injection and post administration monitoring |
12/08/2021 – 01/26/2023 |
M0221 |
Tixagev and cilgav inj hm |
AstraZeneca |
Injection, tixagevimab and cilgavimab, for the pre-exposure prophylaxis only, for certain adults and pediatric individuals (12 years of age and older weighing at least 40kg) with no known sars-cov-2 exposure, who either have moderate to severely compromised immune systems or for whom vaccination with any available COVID-19 vaccine is not recommended due to a history of severe adverse reaction to a COVID-19 vaccine(s) and/or COVID-19 vaccine component(s), includes injection and post administration monitoring in the home or residence; this includes a beneficiary’s home that has been made provider-based to the hospital during the COVID-19 public health emergency |
12/08/2021 – 01/26/2023 |
Q0222**** |
Bebtelovimab 175 mg |
Eli Lilly |
Injection, bebtelovimab, 175 mg |
02/11/2022 – 11/30/2022 |
M0222 |
Bebtelovimab injection |
Eli Lilly |
Intravenous injection, bebtelovimab, includes injection and post administration monitoring |
02/11/2022 – 11/30/2022 |
M0223 |
Bebtelovimab injection home |
Eli Lilly |
Intravenous injection, bebtelovimab, includes injection and post administration monitoring in the home or residence; this includes a beneficiary’s home that has been made provider-based to the hospital during the COVID-19 public health emergency |
02/11/2022 – 11/30/2022 |
Q0239* |
bamlanivimab-xxxx |
Eli Lilly |
Injection, bamlanivimab, 700 mg |
11/10/2020 – 04/16/2021 |
M0239 |
bamlanivimab-xxxx infusion |
Eli Lilly |
Intravenous infusion, bamlanivimab-xxxx, includes infusion and post administration monitoring |
11/10/2020 – 04/16/2021 |
Q0240* |
Casirivi and imdevi 600mg |
Regeneron |
Injection, casirivimab and imdevimab, 600 mg |
07/30/2021 – 01/24/2022 |
M0240 |
Casiri and imdev repeat |
Regeneron |
Intravenous infusion or subcutaneous injection, casirivimab and imdevimab includes infusion or injection, and post administration monitoring, subsequent repeat doses |
07/30/2021 – 01/24/2022 |
M0241 |
Casiri and imdev repeat hm |
Regeneron |
Intravenous infusion or subcutaneous injection, casirivimab and imdevimab includes infusion or injection, and post administration monitoring in the home or residence, this includes a beneficiary's home that has been made provider-based to the hospital during the COVID-19 public health emergency, subsequent repeat doses |
07/30/2021 – 01/24/2022 |
Q0243* |
casirivimab and imdevimab |
Regeneron |
Injection, casirivimab and imdevimab, 2400 mg |
11/21/2020 – 01/24/2022 |
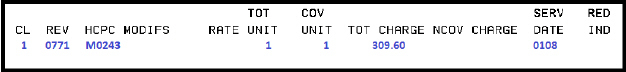
M0243 |
Casirivi and imdevi inj |
Regeneron |
Intravenous infusion or subcutaneous injection, casirivimab and imdevimab includes infusion or injection, and post administration monitoring |
11/21/2020 – 01/24/2022 |
Q0244** |
casirivi and imdevi 1200 mg |
Regeneron |
Injection, casirivimab and imdevimab, 1200 mg |
06/03/2021 – 01/24/2022 |
M0244** |
Casirivi and imdevi inj hm |
Regeneron |
Intravenous infusion or subcutaneous injection, casirivimab and imdevimab includes infusion or injection, and post administration monitoring in the home or residence; this includes a beneficiary’s home that has been made provider-based to the hospital during the COVID-19 public health emergency |
05/06/2021 – 01/24/2022 |
Q0245* |
bamlanivimab and etesevima |
Eli Lilly |
Injection, bamlanivimab and etesevimab, 2100 mg |
02/09/2021 – 01/24/2022 |
M0245 |
bamlan and etesev infusion |
Eli Lilly |
Intravenous infusion, bamlanivimab and etesevimab, includes infusion and post administration monitoring |
02/09/2021 – 01/24/2022 |
M0246** |
bamlan and etesev infus home |
Eli Lilly |
Intravenous infusion, bamlanivimab and etesevimab, includes infusion and post administration monitoring in the home or residence; this includes a beneficiary’s home that has been made provider-based to the hospital during the COVID-19 public health emergency |
05/06/2021 – 01/24/2022 |
Q0247*** |
sotrovimab |
GSK |
Injection, sotrovimab, 500 mg |
05/26/2021 – 04/05/2022 |
M0247 |
sotrovimab infusion |
GSK |
Intravenous infusion, sotrovimab, includes infusion and post administration monitoring |
05/26/2021 – 04/05/2022 |
M0248** |
sotrovimab inf, home admin |
GSK |
Intravenous infusion, sotrovimab, includes infusion and post administration monitoring in the home or residence; this includes a beneficiary’s home that has been made provider-based to the hospital during the COVID-19 public health emergency |
05/26/2021 – 04/05/2022 |
Q0249*** |
Tocilizumab for COVID-19 |
Genentech |
Injection, tocilizumab, for hospitalized adults and pediatric patients (2 years of age and older) with COVID-19 who are receiving systemic corticosteroids and require supplemental oxygen, non-invasive or invasive mechanical ventilation, or extracorporeal membrane oxygenation (ECMO) only, 1 mg |
06/24/2021 |
M0249 |
Adm Tocilizu COVID-19 1st |
Genentech |
Intravenous infusion, tocilizumab, for hospitalized adults and pediatric patients (2 years of age and older) with COVID-19 who are receiving systemic corticosteroids and require supplemental oxygen, non-invasive or invasive mechanical ventilation, or extracorporeal membrane oxygenation (ECMO) only, includes infusion and post administration monitoring, first dose |
06/24/2021 |
M0250 |
Adm Tocilizu COVID-19 2nd |
Genentech |
Intravenous infusion, tocilizumab, for hospitalized adults and pediatric patients (2 years of age and older) with COVID-19 who are receiving systemic corticosteroids and require supplemental oxygen, non-invasive or invasive mechanical ventilation, or extracorporeal membrane oxygenation (ECMO) only, includes infusion and post administration monitoring, second dose |
06/24/2021 |